Help shape the 2025 Lab Efficiency Report!
Take the survey now.
Both LIS and LIMS originated from the same need: to improve lab recordkeeping. Now, even seasoned lab folks can mix them up, and we get why. Their names are almost identical, but these two types of lab software serve different purposes. Find out what each system does, and which one your lab needs (with a decision checklist to make things easier).

If you look up “laboratory information management system” on Wikipedia, you’ll find a slightly confusing note: LIMS, sometimes called a laboratory information system (LIS).

So…the world’s biggest online encyclopedia says LIMS is basically the same as a LIS. Does that mean your lab could use either one? Well, not quite.
We’re not saying Wikipedia is wrong here: a lot of people do mix up LIMS and LIS. That’s exactly why we wrote this article.
Read on for a quick look at the origins of these two lab systems (don’t worry, it won’t be too overwhelming). You’ll see what makes them different, which labs use each (with real-life examples), a side-by-side comparison, and a checklist to help you choose the right fit.
If you’re tracking who is being tested (patients), you need an LIS.
If you’re tracking what is being tested (samples, batches), you need a LIMS.
Let’s say you run a hospital lab, processing bloodwork and sending results to doctors. You need a system that links every result to a patient, updates their electronic health record (EHR), and helps with insurance claims. That’s a job for LIS, a Laboratory Information System.
Now imagine you’re in a quality control lab for a food company, tracking hundreds of samples each day from raw ingredients to finished products. You have to follow strict standards: every test must be done and recorded properly, so nothing unsafe slips through. That’s when you need LIMS, a Laboratory Information Management System.
In short, the “M” for management is what sets these two types of lab software apart.
LIS delivers the right information to the right doctor or patient. It’s built for labs with fixed, routine workflows, like in hospitals or diagnostics.
LIMS helps manage and automate more complex multi-step workflows, like you’ll find in pharma, manufacturing, or research labs.
Let’s go deeper into the topic (warning: a few mildly boring history facts ahead).
Origin: LIS appeared in the late 1960s, when hospital labs needed a way to log and share test results electronically. They followed strict, repetitive workflows set by healthcare law and hospital SOPs. LIS just had to record what happened and get the results where they needed to go. It remains the same way now.
Note: Many LIS now have “management” features, too (scheduling, automation, etc.), but the core mission is still to deliver patient information, not help run the lab.
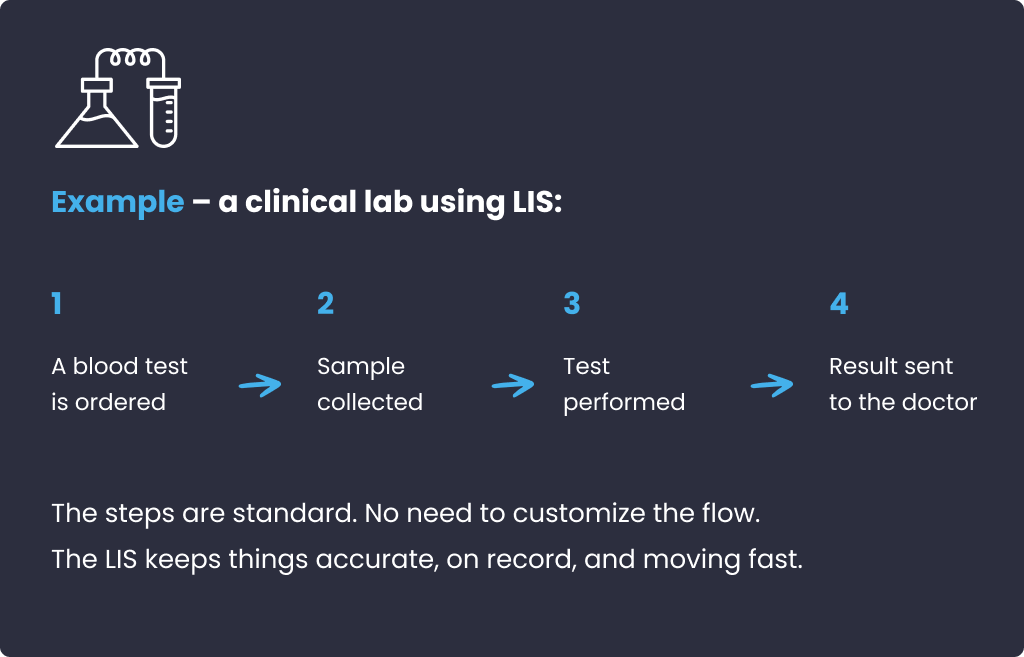
Origin: LIMS came a bit later, around 1982, offering automated reporting tools. It came out of labs that had more steps, more complexity, and way more paperwork, like in pharma, food safety, or materials testing.
Fun fact: Some labs rely on LIMS so much that “LIMS Admin” is now a real job – part lab tech, part QA, part IT support:
So why the “Management” part? Because labs didn’t just need to log results. They needed help running the whole operation. Think of receiving materials, batch processing, multiple rounds of QC, approvals, instrument scheduling, chain of custody, compliance docs... and the steps often change depending on the product, client, or test type.
By the way, our own 1LIMS is a LIMS platform (shocking, we know 😄) built exactly for this kind of work. Since 2016, we’ve been helping European manufacturers and lab teams move ditch the chaos and automate QC/QA. Or as one of our clients put it:

That’s enough history for now. Let’s switch gears and look at where LIS and LIMS are actually used, starting with real labs and real examples.
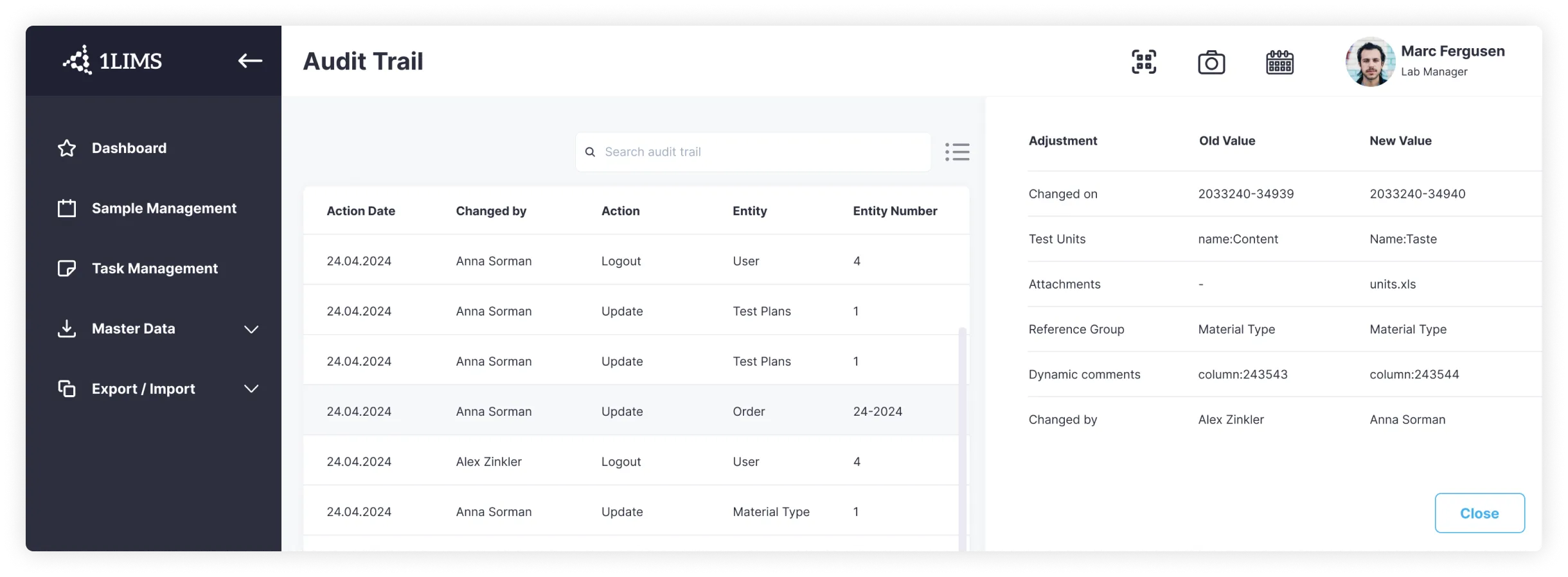
When it comes to laboratory information management systems (LIMS) and laboratory information systems (LIS), robust data management and data integrity are non-negotiable. These systems are the backbone of modern labs, handling everything from patient data and sample tracking to test results and inventory management.
In a LIS, the focus is on managing patient-centric data. This means every piece of information—medical history, test results, prescriptions, and more—must be accurately linked to the right patient. The system ensures that sensitive patient data is securely stored and easily accessible for clinical decision-making, while also supporting compliance with healthcare regulations.
On the other hand, LIMS is all about sample-centric data management. Here, the priority is accurate sample tracking, from collection through testing and reporting. LIMS streamlines the testing process, manages inventory, and keeps a detailed record of every sample’s journey through the lab. This is essential for labs handling high volumes of samples, such as those in environmental testing or manufacturing.
Both LIS and LIMS are designed to uphold data integrity at every step. They use advanced security features like access controls, encryption, and audit trails to protect laboratory data from unauthorized access or accidental errors. By maintaining strict data integrity, these systems ensure that testing results are reliable and trustworthy—critical for both patient care and research outcomes.
In short, whether your lab is managing patient-centric or sample-centric data, choosing the right system is key to ensuring smooth laboratory operations, accurate test results, and full compliance with industry standards.
LIS is for labs that deal with patient data. Think hospital bloodwork, where every test is tied to a specific person. A LIS keeps track of every test, order, and result – and makes sure they’re correctly linked to medical records and billing.
✔️ Integrations: A LIS needs to sync with EHRs, insurance, and hospital systems via HL7 or FHIR standards so doctors can see results right away.
✔️ Compliance: LIS is built with healthcare laws in mind, like HIPAA, CLIA, and CAP.
Here are the labs where you’ll find LIS in place.
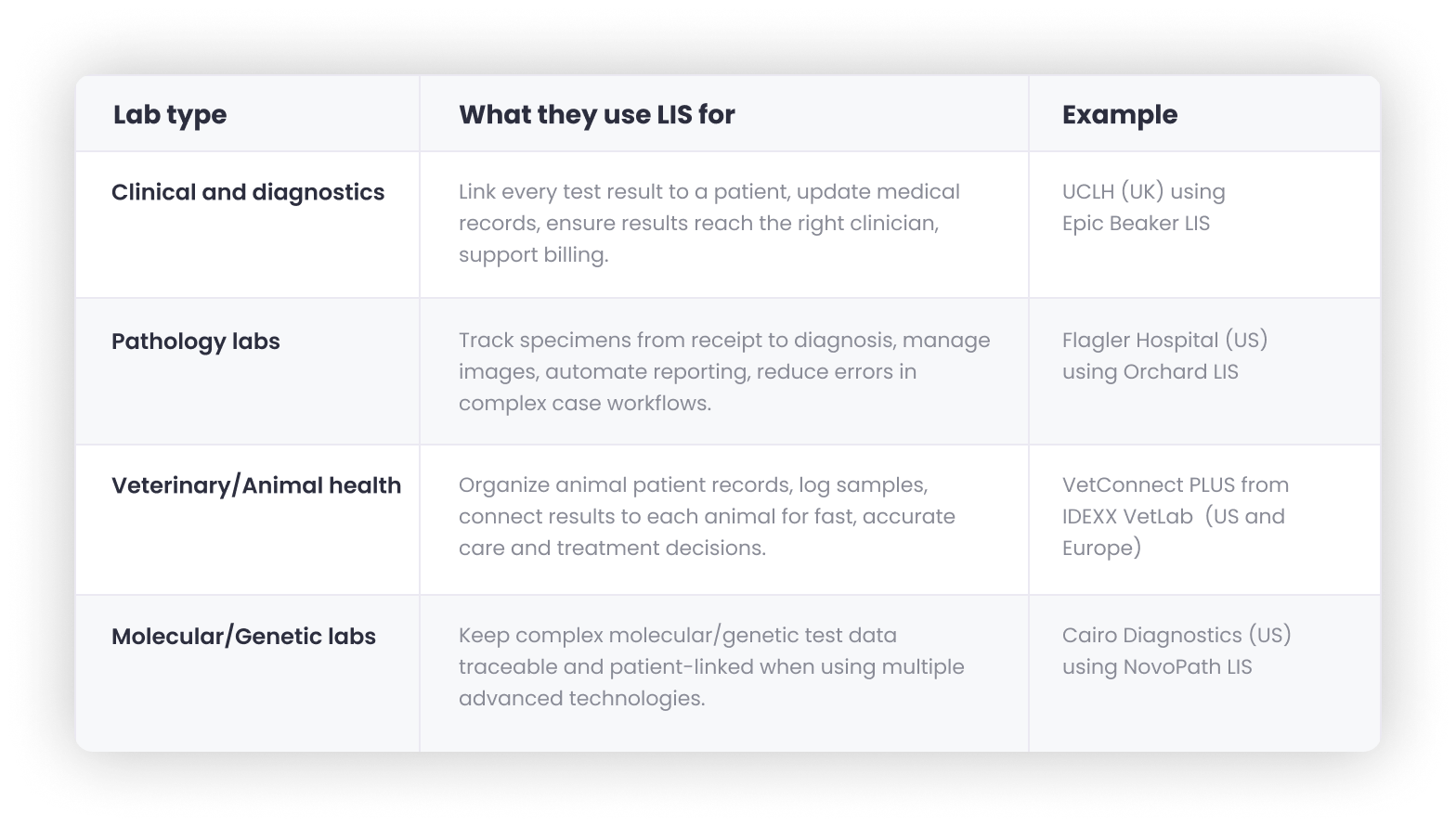
🏥 LIS is for labs that manage patient data. It must connect with EHRs, billing, and healthcare systems and comply with HIPAA, CLIA, CAP, and healthcare data laws.
These labs use LIS to match each test result with the right patient and send it to the right doctor. LIS also helps keep medical records current for ongoing care.
👉 Case in point:
University College London Hospitals (UCLH), one of the UK’s largest teaching hospitals, uses Epic Beaker LIS to link diagnostics, pathology, and patient records across the NHS. Every result is tied to the patient’s record so clinicians can act fast and bill accurately.
Pathology labs rely on LIS to track specimens from receipt through diagnosis, manage digital images, and keep the entire process traceable.
👉 Case in point:
Flagler Hospital’s pathology lab in the US uses Orchard® Enterprise Pathology™ LIS to manage every step of the pathology process, flag urgent cases, and assign work to pathologists automatically.
LIS also works for animal patients. It keeps each pet or livestock case organized, ties test results to the right patient, and speeds up turnaround.
👉 Case in point:
VetConnect PLUS from IDEXX VetLab acts as a LIS platform for thousands of animal clinics across Europe and North America. It lets vets track samples, order tests, and get results fast, all tied to each animal’s file.
These labs often run complex tests, like NGS, PCR, or FISH, that must stay connected to the right case and patient throughout. A LIS keeps everything in sync.
👉 Case in point:
Cairo Diagnostics, a hematopathology lab, uses NovoPath’s LIS to manage traditional and molecular test data. It tracks specimens, connects every test to the right patient, and creates one complete diagnostic report.
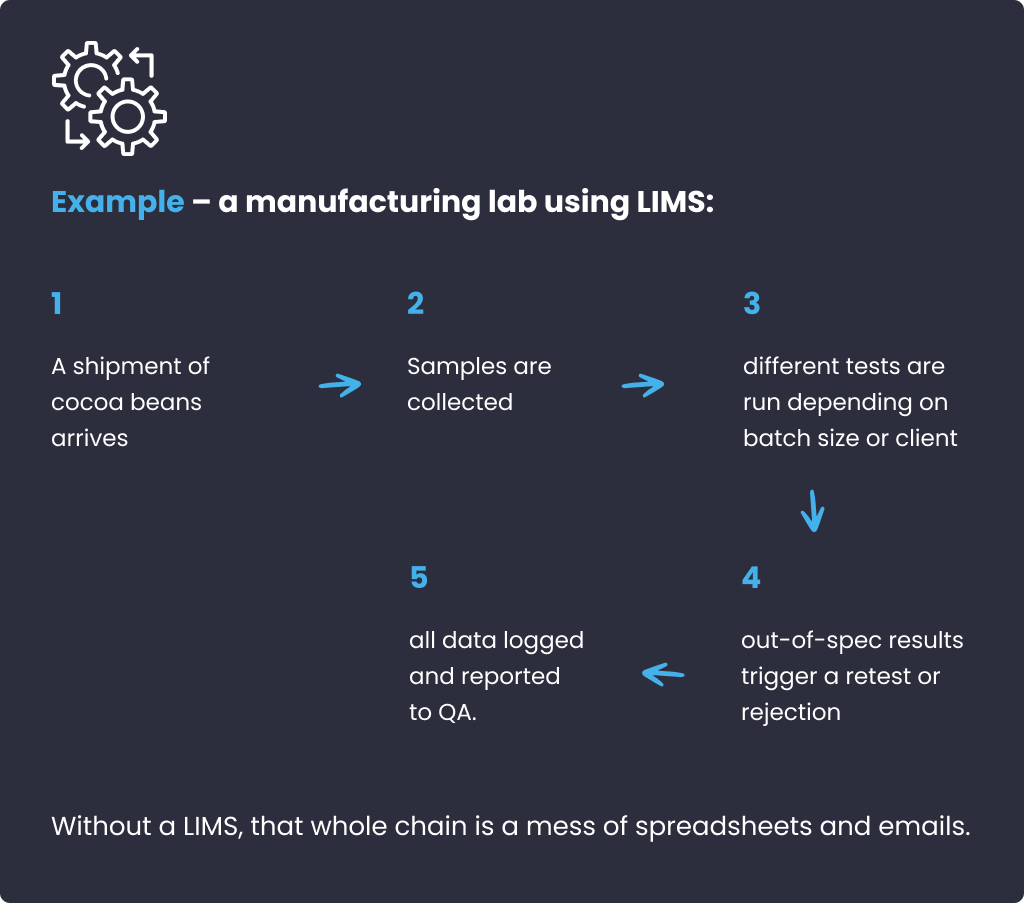
LIMS is for labs that track samples, batches, or materials, not people. Think logging a shipment of cocoa beans and tracking every test until the final chocolate bar. LIMS helps you barcode samples, automate test scheduling, and generate Certificates of Analysis, so nothing gets missed and everything is traceable.
✔️ Integrations: LIMS connects with lab instruments, ERPs, and supply chain tools.
✔️ Compliance: LIMS is built with industry regulations in mind, like FDA, GLP, ISO 17025
Here’s where LIMS is used, with real-world examples, some from our clients:
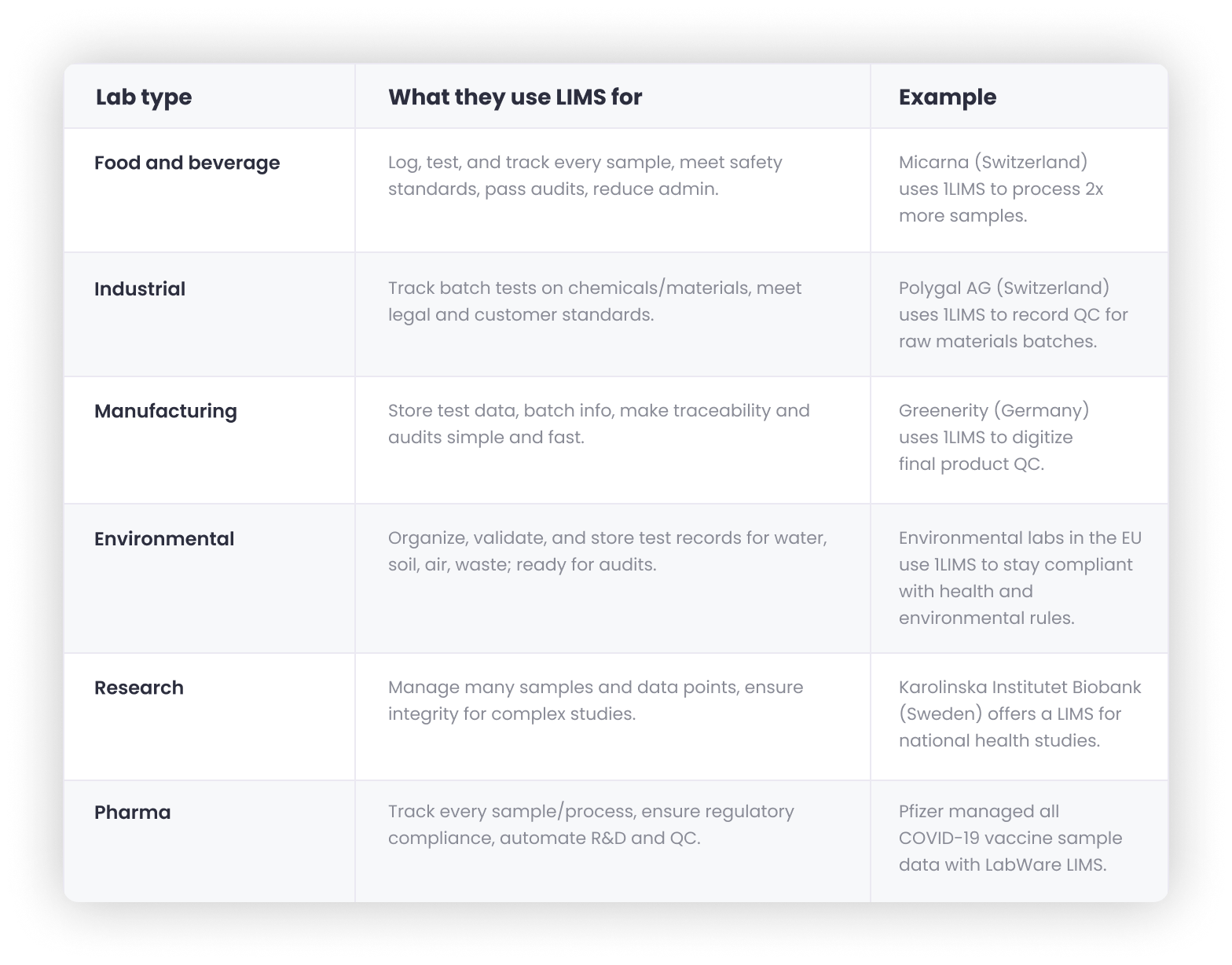
🏭 LIMS is for labs tracking samples, batches, or materials. It must connect with lab instruments, ERPs, supply chain tools, and external service laboratories and comply with FDA, GLP, ISO 17025, and industry standards.
These labs test meat, drinks, and other food products. They use LIMS to log, test, and trace every sample, keeping quality high and audits stress-free.
👉 Case in point:
Micarna, one of Switzerland’s top meat producers and a longtime 1LIMS client, now processes 50–60% more samples and breezes through audits that used to be a “nightmare”.

Industrial labs test chemicals, materials, and ingredients. LIMS helps them track and standardize results across every batch.
👉 Case in point:
Polygal AG is a Swiss company that’s been producing natural hydrocolloids, ingredients that end up in food, pharma, and cosmetics. They used to juggle MS Access and spreadsheets – “a huge pain,” according to lab manager Sindy Seidel. “It ate up a ton of time and was always prone to errors.” Now, with 1LIMS, they’ve automated their lab workflows and have everything in one place. They got it all up and running in just one month.
Manufacturing labs use LIMS to log every test and keep track of batches. That way, if clients or inspectors have questions, the answers are easy to find.
👉 Case in point:
Greenerity, a leading German hydrogen and hydro cell company founded in 2006, wanted to upgrade their product safety system. Before going all in, they asked 1LIMS to assess their lab processes. Through our signature LabCheck workshop, we found they could cut lab workload in half and save EUR 41,000 per year. With that clarity, Greenerity moved forward with digitalizing their lab ops using 1LIMS.
🤔 Want to know how your lab measures up to best practices?
Take our quick LabCHECK to see where you stand, and get a free Lab Digitalization Guide based on your answers.
Environmental labs test water, soil, air, and waste. LIMS helps them stay audit-ready and compliant with EU and global environmental standards.
👉 Case in point:
1LIMS supports several environmental testing labs across Europe to manage and trace all sample and test records, ensuring they’re fully prepared for audits.
Research labs turn to LIMS to manage large collections of samples, track every data point, and maintain integrity across complex studies.
👉 Case in point:
The Karolinska Institutet Biobank in Sweden offers a LIMS to help research groups manage data for projects like LifeGene and EpiHealth, with tens of thousands of unique samples and complex genotype data.
Pharma labs rely on LIMS to meet strict compliance rules (FDA, GLP), track every sample and test, and automate data management across R&D, QA, and manufacturing.
👉 Case in point:
Pfizer uses LabWare LIMS to manage every sample during drug development and production. For example, their COVID-19 vaccine batches were tracked end-to-end, from cell growth to final release, with LIMS keeping tabs on location, results, and approvals.
Bottom line
If the lab’s main question is “What are we testing?” → they use LIMS.
If it’s “Who are we testing?” → they turn to LIS.
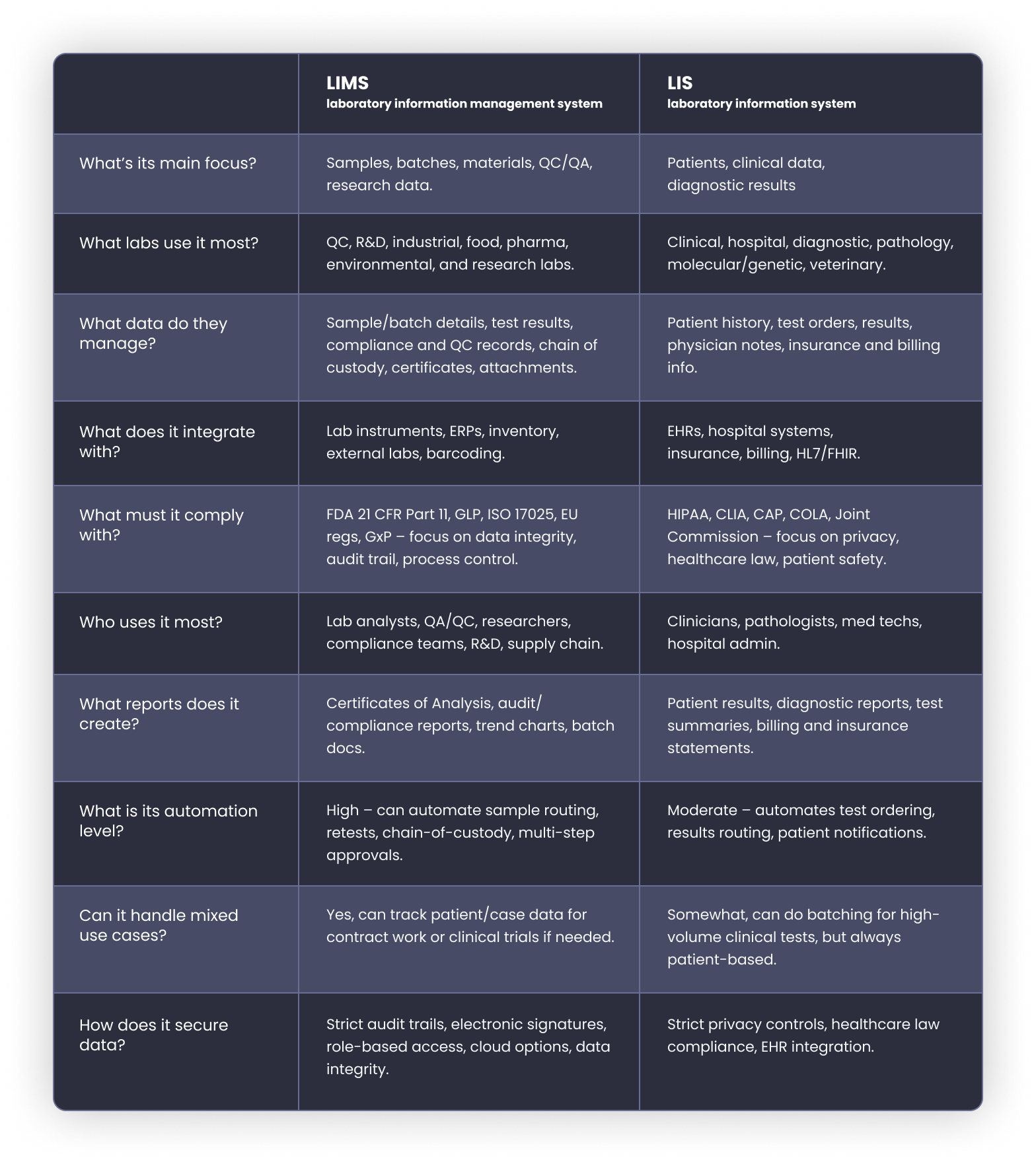
Now, let’s see how both these types of lab software compare feature by feature.
LIS and LIMS’ core features serve different lab realities. Here’s how they compare when you look under the hood:
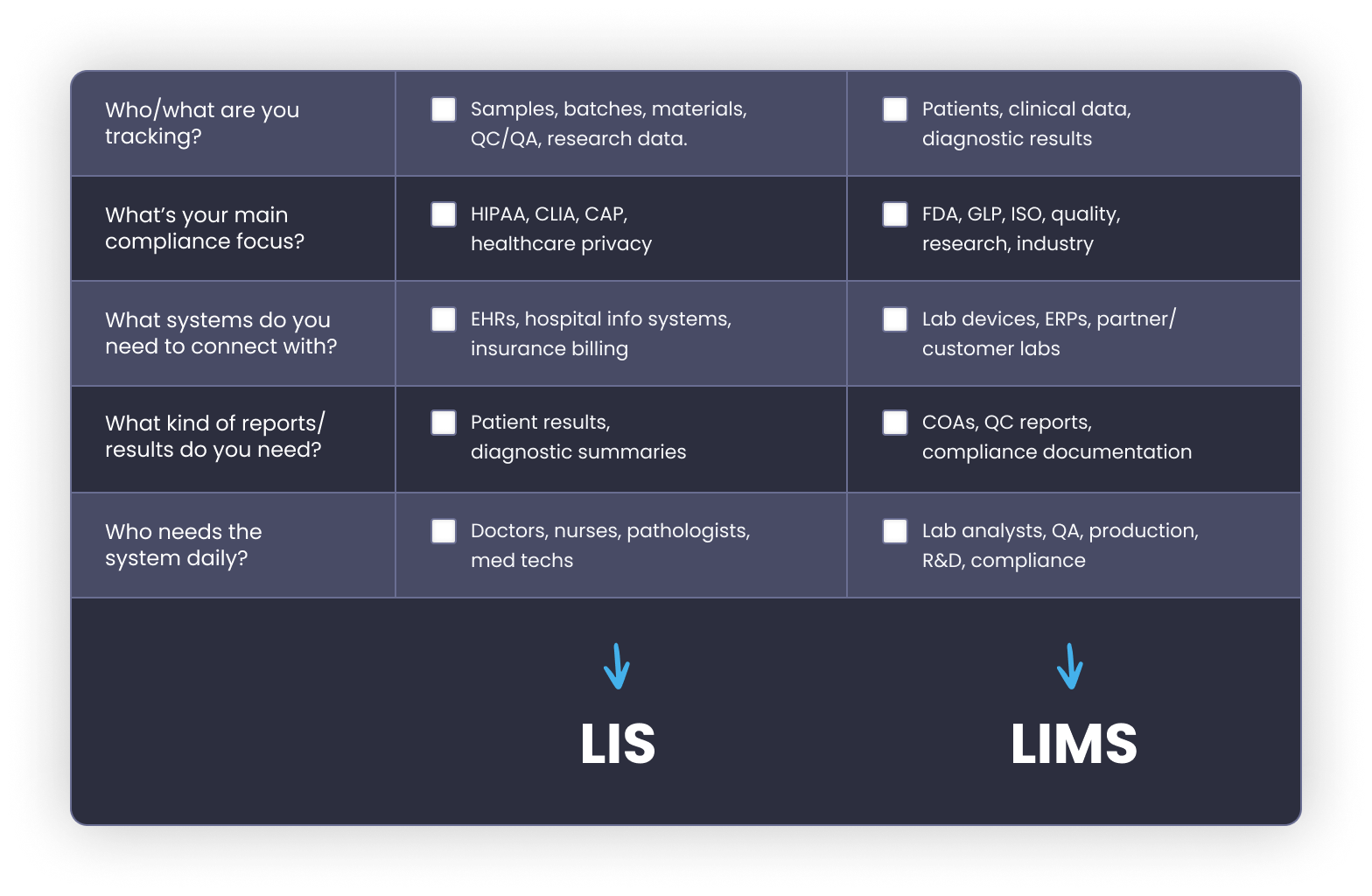
Alright, we’ve made it this far, so let’s bring it home. Here’s a simple checklist to help you figure out what kind of system your lab actually needs: LIS or LIMS.
✔️ If you check LIS for 3 or more → your lab probably needs an LIS.
✔️ If you check LIMS for 3 or more → you’re likely in LIMS territory.
Note: When in doubt, focus on what your auditors require and how your team actually works day to day.
Pro tip: When selecting software, ask vendors to walk through a real-life scenario from your lab, not just a polished demo with dummy data. Try something like:
“Show me how your LIS would handle a leukemia patient who gets bloodwork on Monday, a bone marrow biopsy on Wednesday, and genetic testing next week. Can your system tie all that together into one report for the hematologist?”
That kind of test tells you way more than any feature list.
Adopting a laboratory information system (LIS) or laboratory information management system (LIMS) can be a game-changer for any lab. These laboratory software systems are designed to boost efficiency, enhance productivity, and take the headache out of data management.
For clinical labs, LIS streamlines workflows by automating everything from test ordering to results reporting. This means faster turnaround times, fewer manual errors, and improved patient care. With seamless integration to electronic health records (EHRs) and lab instruments, LIS ensures that patient data flows smoothly between systems, supporting accurate diagnoses and billing.
LIMS, meanwhile, is a powerhouse for labs focused on sample tracking and complex laboratory operations. It optimizes every step of the process-logging samples, scheduling tests, managing inventory, and analyzing data. LIMS makes it easy to handle large volumes of research data, automate routine tasks, and generate detailed reports for quality control and compliance.
Both LIS and LIMS help labs reduce administrative burdens, minimize errors, and improve data analysis. By connecting with other software systems and lab instruments, they create a unified, efficient workflow that supports high-quality results and better decision-making.
In the end, implementing a laboratory information system isn’t just about keeping up with technology - it’s about transforming your lab’s performance, cutting costs, and delivering better outcomes for patients and clients alike.
Rolling out a new laboratory information management system (LIMS) or laboratory information system (LIS) is a big step, but with the right approach, it can be a smooth transition that pays off quickly. Here’s what labs can expect during implementation and integration.
First, there’s a thorough assessment of your current workflows, data management practices, and existing software systems. This helps tailor the new system to your lab’s unique needs, whether you’re focused on patient data, sample tracking, or both.
Next comes configuration-setting up the laboratory information system LIS or LIMS to match your processes, from test ordering to reporting. Integration is a critical phase: connecting the new system with lab instruments, electronic health records, and other software systems ensures seamless data flow and enables workflow automation.
Training is another key step. Lab staff need to feel confident using the new system, so hands-on training and support are essential. After go-live, expect ongoing maintenance and updates to keep everything running smoothly and securely.
A successful implementation is a team effort, involving lab technicians, IT professionals, and your software vendor. With careful planning and collaboration, your lab can achieve faster, more accurate data management and unlock the full potential of your new system.
To get the most out of your laboratory information management systems or laboratory information systems, it’s important to follow a set of best practices that keep your lab running smoothly and your data secure.
Start with regular system maintenance,keep your software up to date and perform routine checks to catch any issues early. Ongoing training for lab staff ensures everyone is comfortable with the system and aware of any new features or updates.
Establish clear policies for data management and system use, including who can access what information. Implement strong access controls, encryption, and audit trails to protect data integrity and prevent unauthorized access. Regular backups and a solid disaster recovery plan are must-haves to safeguard against data loss.
Don’t forget compliance: schedule regular audits to ensure your lab meets all regulatory requirements, whether you’re governed by HIPAA, CLIA, or industry-specific standards. By prioritizing data integrity, accuracy, and security, your lab can maintain high-quality operations and deliver reliable results every time.
The world of laboratory information management systems and laboratory information systems is evolving fast, with exciting developments on the horizon. Today’s LIS and LIMS are becoming smarter, more flexible, and more connected than ever before.
Artificial intelligence (AI) and machine learning (ML) are starting to play a bigger role, helping labs analyze complex data sets and make faster, more accurate decisions. Cloud-based systems are gaining traction, offering labs greater scalability, easier updates, and cost savings - plus the ability to access data from anywhere.
Mobile devices and wearable tech are also making their way into the lab, allowing staff to manage data and monitor laboratory operations on the go. As personalized medicine grows, LIS and LIMS are adapting to handle new types of data, like genomics and lifestyle information, to support tailored treatment plans.
Looking ahead, the integration of advanced analytics, improved interoperability, and user-friendly interfaces will continue to drive innovation. As technology advances, LIS and LIMS will remain at the heart of efficient lab operations, supporting better patient care, groundbreaking research, and the future of healthcare.
If your answers lean towards LIMS, we can help. At 1LIMS, we work with labs like yours to automate workflows, streamline sample tracking, and connect everything – ERP, MES, CRM, lab devices, and external service providers.
And one more thing: you can go live with 1LIMS in just 30 days, like Polygal AG and many of our clients did. (Compare that to the 6–12+ months it takes with many traditional LIMS setups.)

Worried digitalizing your lab might be too much? Start small: take our quick 10-question LabCHECK quiz. You’ll get a personalized Digitalization Guide that shows where you stand – and how to move forward, one step at a time. Orbook a demo and let us show you around 1LIMS. See you around!